Ehanire said it was important for NAFDAC to approve the vaccines
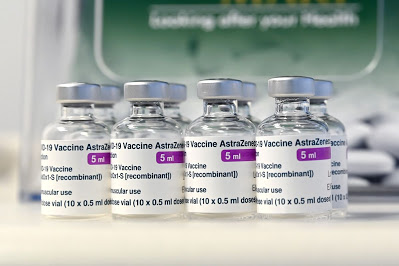
The National Agency for Food and Drug Administration and Control (NAFDAC) has approved Oxford/AstraZeneca COVID-19 vaccine for use in Nigeria.
The director-general of NAFDAC, Mojisola Adeyeye, disclosed this during a media briefing on Thursday.
The minister of health, Osagie Ehanire, had on Monday said the federal government had sent vaccine dossiers of Sputnik V from Russia and AstraZeneca manufactured by the Serum Institute of India for evaluation and approval by NAFDAC.
Ehanire said it was important for NAFDAC to approve the vaccines because “all COVID-19 vaccines carry a certain amount of risks and any vaccines not approved” cannot be used in Nigeria.
NAFDAC DG, during the media briefing, informed that there were three additional vaccines undergoing evaluation. She said the evaluation of the AstraZeneca vaccine shows that it is effective against the UK variant of the virus-B117- which has also been found in Nigeria.
The AstraZeneca vaccine was approved by the World Health Organisation (WHO) for emergency use on Monday.
In its review, WHO said the AstraZeneca vaccine met the “must-have” criteria for safety, and its efficacy benefits outweighed its risks.
The Oxford/AstraZeneca vaccine, also known as ChAdOx1 nCoV-19, or AZD1222, is a viral vector vaccine.










![Restoring Vision, Transforming Lives: OGD’s Free Eye Surgery Initiative Reaches a New Milestone! [Video]](https://newsheadline247.com/wp-content/uploads/2023/07/Gbenga_Daniel_newsheadline247-1-200x150.jpg)












